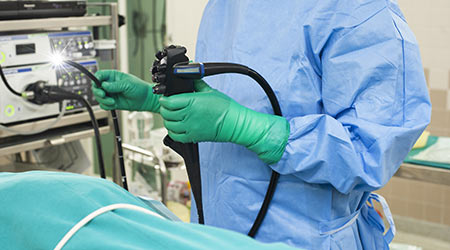
Preliminary results from the US Food and Drug Administration's (FDA's) mandated postmarket surveillance studies of duodenoscopes show "higher-than-expected" contamination rates after reprocessing, according to an article on the Medscape website.
The FDA said 3 percent of properly collected samples tested positive for more than 100 colony-forming units of "low-concern" organisms.
These organism are unlikely to cause serious infections but are, nevertheless, an indication of a "reprocessing failure."
An additional 3 percent of properly collected samples tested positive for "high-concern" bacteria that are more often associated with disease.
 Design Plays a Role in the Future of Healthcare
Design Plays a Role in the Future of Healthcare Cedar Hill Regional Medical Center GW Health Officially Opens
Cedar Hill Regional Medical Center GW Health Officially Opens Designing Healthcare Facilities for Pediatric and Geriatric Populations
Designing Healthcare Facilities for Pediatric and Geriatric Populations Kaiser Permanente Announces New Hospital Tower at Sunnyside Medical Center
Kaiser Permanente Announces New Hospital Tower at Sunnyside Medical Center Building Disaster Resilience Through Collaboration
Building Disaster Resilience Through Collaboration