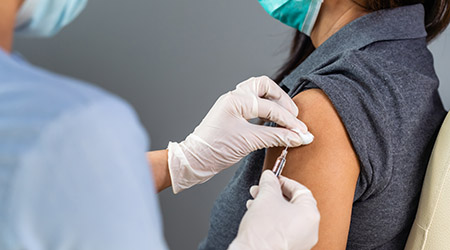
The Centers for Medicare & Medicaid Services (CMS) is requiring COVID-19 vaccination of eligible staff at health care facilities that participate in the Medicare and Medicaid programs. The emergency regulation aims to protect front-line workers fighting this virus while also delivering assurances to individuals and their families that they will be protected when seeking care.
These requirements will apply to approximately 76,000 providers and cover over 17 million health care workers across the country. The regulation will create a consistent standard within Medicare and Medicaid while giving patients assurance of the vaccination status of those delivering care.
The interim final rule establishes a Condition of Participation that applies to most health care settings, including hospitals, critical access hospitals, ambulatory surgery centers, comprehensive outpatient rehabilitation facilities, home health agencies, rural health clinics, federally qualified health centers and long-term care facilities. The vaccination requirement applies to all eligible staff working at a facility that participates in the Medicare and Medicaid programs, regardless of clinical responsibility or patient care, including staff who work in offsite locations, such as homes, clinics or administrative offices. The requirement does not apply to individuals who provide services 100 percent remotely and have no direct contact with patients and other staff.
Facilities covered by this regulation must establish a policy ensuring all eligible staff have received the first dose of a two-dose COVID-19 vaccine or a one-dose COVID-19 vaccine prior to providing any care, treatment, or other services by December 5, 2021. All eligible staff must have received the necessary shots to be fully vaccinated – either two doses of Pfizer or Moderna or one dose of Johnson & Johnson – by Jan. 4, 2022. The regulation also provides for exemptions based on recognized medical conditions or religious beliefs, observances, or practices. Facilities must develop a similar process or plan for permitting exemptions in alignment with federal law.
 Mature Dry Surface Biofilm Presents a Problem for Candida Auris
Mature Dry Surface Biofilm Presents a Problem for Candida Auris Sutter Health's Arden Care Center Officially Opens
Sutter Health's Arden Care Center Officially Opens Insight Hospital and Medical Center Falls to Data Breach
Insight Hospital and Medical Center Falls to Data Breach The High Cost of Healthcare Violence
The High Cost of Healthcare Violence EVS Teams Can Improve Patient Experience in Emergency Departments
EVS Teams Can Improve Patient Experience in Emergency Departments