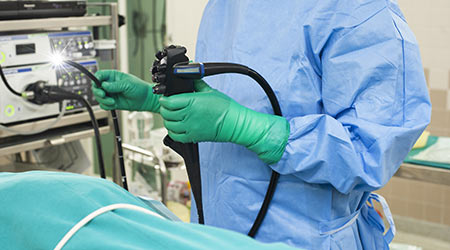
Preliminary results from the US Food and Drug Administration's (FDA's) mandated postmarket surveillance studies of duodenoscopes show "higher-than-expected" contamination rates after reprocessing, according to an article on the Medscape website.
The FDA said 3 percent of properly collected samples tested positive for more than 100 colony-forming units of "low-concern" organisms.
These organism are unlikely to cause serious infections but are, nevertheless, an indication of a "reprocessing failure."
An additional 3 percent of properly collected samples tested positive for "high-concern" bacteria that are more often associated with disease.
 All Eyes on Gen Z as They Enter the Workforce
All Eyes on Gen Z as They Enter the Workforce Cleveland Clinic Starts Fundraising Effort for New Hospital in West Palm Beach
Cleveland Clinic Starts Fundraising Effort for New Hospital in West Palm Beach Cultivating a Workforce in the Face of Skilled Trade Shortages
Cultivating a Workforce in the Face of Skilled Trade Shortages Prime Healthcare Acquires 8 Ascension Hospitals in Illinois
Prime Healthcare Acquires 8 Ascension Hospitals in Illinois Charging Ahead: Incentives Driving EV Charging in Healthcare Facilities
Charging Ahead: Incentives Driving EV Charging in Healthcare Facilities