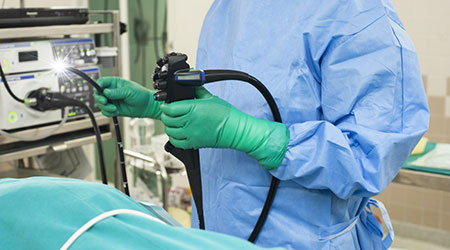
The Food and Drug Administration (FDA) said companies that make duodenoscopes should begin making disposable versions, according to an article on The New York Times website.
The agency also said that hospitals should start to transition to models with disposable components to reduce the risk of infection.
FDA tests revealed that one in 20 harbored disease-causing microbes like E. coli even after being properly cleaned.
Medical experts have urged the FDA to force manufacturers to make the scopes safer and easier to decontaminate — or to take them off the market.
 3 Employees Injured by Patient at Halifax Infirmary's Emergency Department
3 Employees Injured by Patient at Halifax Infirmary's Emergency Department How Architects Shape the Future of Healthcare Facilities
How Architects Shape the Future of Healthcare Facilities UNC Health, Duke Health Form Partnership for Stand-alone Children's Hospital
UNC Health, Duke Health Form Partnership for Stand-alone Children's Hospital Sarasota Memorial Hospital Plans to Build New Facility in North Port
Sarasota Memorial Hospital Plans to Build New Facility in North Port CMMS, Data and the Path to Compliance
CMMS, Data and the Path to Compliance