Creating and manufacturing medical devices requires adherence to extensive regulatory standards, research and testing as well as accurate performance analytics to improve the process. As the healthcare industry continues to evolve, regulations and processes will evolve as well, helping to ensure the patient’s safety and the nation’s health.
Evolving government regulation
As the governing body for public health and medical devices, the Food and Drug Administration (FDA) is working to build a stronger system using data to test and improve patient care. By analyzing real-world evidence, the FDA can guide toward improved device safety and innovation. While continuing to innovate, it’s critical that original equipment manufacturers (OEMs) consider what materials are being used in their products to ensure successful devices.
The regulation of medical devices is a complex process because devices range from tongue depressors to life-saving heart valves. Classified by the risk they pose to consumers, devices must be registered with the FDA and follow general requirements as written in the Federal Food, Drug and Cosmetic Act (FFDCA). Manufacturers have two options when bringing products to the market. They can conduct clinical studies, provide evidence that their device is effective and safe and get premarket approval (PMA). The other option requires OEMs to submit a 510k notification showing that the device is equivalent to a device already on the market or predicate device. This does not require a PMA and is a much simpler process. Performance characteristics are compared to the predicate device and analyzed.
To generate better evidence for regulatory decision-making and medical device evaluation, the FDA is building the National Evaluation System for Health Technology (NEST). NEST will follow the entire product lifestyle of medical devices by using real-world evidence and applying advanced analytics. The system will lead to better informed treatment decisions and find balance between safety and device innovation. NEST has been a priority for the FDA and will ultimately undertake the following activities:
• Promote implementation of Unique Device Identifiers (UDI) into healthcare systems that will be used in device evaluation.
• Fund portfolio activities to support NEST development and implementation through a series of grants.
• Issue guidance to clarify how real-world evidence can be used to support pre- and post-market regulatory decisions.
• Work with medical device ecosystem including federal partners, healthcare system, manufacturers, payers and patients to develop NEST.
Preventing healthcare associated infections
Patients rightly want the best possible healthcare. They want to have confidence in the clinicians and devices that treat them and determine illness. To provide a high level of care and diagnostics, many factors must be considered and although it may seem granular, this can come down to the materials that make up the devices. The materials must be able to withstand years in harsh medical environments with repeated exposure to disinfecting chemicals necessary to combat healthcare-associated infections (HAIs).
Eastman is constantly developing and testing new polymers for use in medical settings. It evaluates the long-term resistance of polycarbonate among other plastics to hospital disinfectants using its 4-step test. In addition, the simple drop test is used for electronic medical device housings to investigate how high stress areas perform after being disinfected and dropped. Eastman shares these simple screening tests with OEMs enabling them to conduct their own evaluations and validate the polymers being used in their devices. Choosing a polymer for use in a medical device that can withstand aggressive drugs, lipids and disinfectant protocols increases infection prevention and control (IPC) and results in an improved hospital experience.
Manufacturing with patients in mind
In the same way that drug manufacturers must use Good Manufacturing Practice (GMP) when producing a drug, so must medical device manufacturers. Regulation requires domestic and foreign manufacturers to manufacture high quality systems throughout the production process and afterward for servicing. Quality systems must have essential elements, but manufacturers still have a great amount of freedom to make quality systems that best cover nuances in their devices.
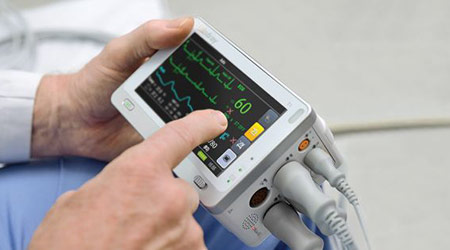
Mindray chose to incorporate Eastman’s medical polymers into its current generation of patient monitors firstly because Eastman was willing to provide real test data that could enhance their testing and selection efficiency. These monitors, used in healthcare facilities throughout North America are called the Passport Series of bedside monitors. The Passport 17m,12m,12,8, the T1 and the Accutorr 3/7 spot check monitors are now fully compatible with a list of approximately 50 of today’s most commonly used cleaners and disinfectants in use due to the increased emphasis on infection prevention control (IPC).
Analyzing the numbers
To deal with these types of issues, in 2019 experts suggest that health systems will have a strong focus on in-house analytics to make the needed improvements. Rather than using big data, small data will be used to leverage existing findings and advance clinical and operational processes. Clinical data is also likely to progress due to better electronic health records (EHRs), leading to additional results and insights.
The shift in analyzing data will lead to a high number of specialty-specific analytics solutions allowing for the introduction of more durable, lasting medical devices. Focused analytics can prove that polymer selection is critical and results in saved costs and best outcomes for patients. Healthcare providers do not have to worry about the impact of using a wide range of disinfectants or accidentally dropping devices. The focus can be on the patient versus issues that are associated with failing devices.
Healthcare payers and providers will also have new goals including leveraging analytics capabilities for population health management, treatment pathways and operational automation. The number one funding category in 2018 was data analytics and it is likely that 2019 will bring advanced demonstrations.
Ellen Turner is Eastman's global market development manager, Specialty Plastics in Medical Devices.
 UF Health Hospitals Rely on Green Globes to Realize Their Full Potential
UF Health Hospitals Rely on Green Globes to Realize Their Full Potential How Healthcare Facilities Can Be Truly Disaster-Resilient
How Healthcare Facilities Can Be Truly Disaster-Resilient TriasMD Breaks Ground on DISC Surgery Center for San Fernando Valley
TriasMD Breaks Ground on DISC Surgery Center for San Fernando Valley Bigfork Valley Hospital Falls Victim to Data Breach
Bigfork Valley Hospital Falls Victim to Data Breach AI-Driven Facilities: Strategic Planning and Cost Management
AI-Driven Facilities: Strategic Planning and Cost Management