In a recent Q&A on the FacilityCare website, consultant Brad Keyes answered a question about humidity and temperature in central supply.
Q: I’m working with an organization on their temperature and humidity monitoring in their central supply area and was wondering what specific parameters they should follow in the two different areas of central supply/storage and decontamination? Should the FGI Guidelines or AAMI Guidelines be followed?
A: If the organization is CMS-certified, all CMS requires on the subject is found in standard §482.41(c)(4), which says, “There must be proper ventilation, light, and temperature controls in pharmaceutical, food preparation, and other appropriate areas.” In its Interpretive Guidelines section, CMS identifies the FGI Guidelines as an acceptable standard to incorporate. While CMS does reference other standards such as AORN, OSHA, CDC and NIH, it does not mention AAMI. So, it would seem acceptable to use the FGI Guidelines in this application. Could your client use AAMI guidelines instead? Yes, but they may have to defend this decision (through the use of risk assessments) to surveyors and inspectors.

 Regulations Take the Lead in Healthcare Restroom Design
Regulations Take the Lead in Healthcare Restroom Design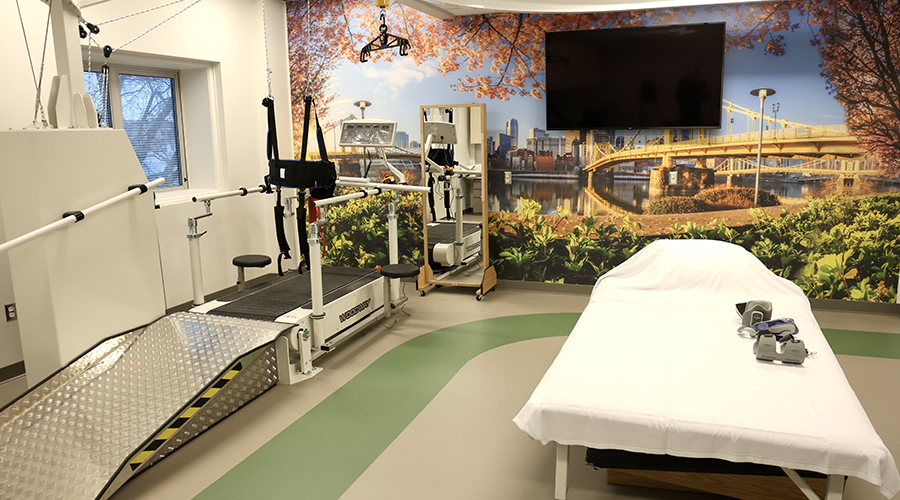 AHN Allegheny Valley Hospital Opens Expanded Inpatient Rehabilitation Unit
AHN Allegheny Valley Hospital Opens Expanded Inpatient Rehabilitation Unit HSHS and Lifepoint Rehabilitation Partner on New Inpatient Rehab Hospital in Green Bay
HSHS and Lifepoint Rehabilitation Partner on New Inpatient Rehab Hospital in Green Bay Turning Facility Data Into ROI: Where Healthcare Leaders Should Start
Turning Facility Data Into ROI: Where Healthcare Leaders Should Start Sutter Health Breaks Ground on Advanced Cancer Center and Care Complex
Sutter Health Breaks Ground on Advanced Cancer Center and Care Complex