Healthcare-associated infections are a major, preventable threat to patient safety and one of the leading causes of death in the United States. According to the Centers for Disease Control (CDC), on any given day, approximately 1 in 25 hospital patients have at least one healthcare-associated infection. This adds up to $33 billion in excess medical costs every year.
In efforts to increase infection control and reduce the risk of hospital-associated infections, regulatory agencies such as The Joint Commission have recommended standards of practice for hospital accreditation. Medicare has implemented financial incentives to reduce and eliminate health-associated infections. In addition, the more recent introduction of state-mandated public disclosure of infection rates has increased accountability through consumer awareness and engagement.
According to some experts, these efforts are not enough as there are currently no national standards for reporting hospital infections. Research from John Hopkins suggests the current system of reporting healthcare quality is non-standardized and haphazard. Claiming most states do not have a standardized system for the collection of data, which leads to great variation in the results making comparisons difficult if not invalid. Many believe the country would be better served by a national standard for hospital infections, and greater transparency would help lower infection rates.
Guidelines and protocols
In the past couple of decades, healthcare delivery has seen a significant shift from the acute inpatient hospital setting to outpatient or ambulatory settings. Nearly, 75% of all operations in the United States are now performed on an outpatient basis. Despite the debate over federal and state regulations, healthcare-associated infections are preventable and prevention should remain a priority in every patient care setting, regardless the level of healthcare provided.
The CDE has provided guidelines to infection prevention for outpatient settings which include: hospital outpatient clinics, non-hospital clinics, physician offices, urgent care centers, surgical centers, public health clinics, imaging centers, oncology clinics, behavioral health clinics, substance abuse clinics, physical therapy and rehabilitation centers.
Infection control protocols should mirror the guidelines and standards set forth by the CDC. All of the following key points should be addressed when policies and procedures for infection control protocol are developed:
• Administration should provide financial and human resources, including equipment and supplies to maintain proper infection prevention. Those with primary administrative oversight should ensure that sufficient fiscal and human resources are available to develop and maintain infection prevention and occupational health programs. This includes the availability of sufficient and appropriate equipment and supplies necessary for the consistent observation of Standard Precautions, including hand hygiene products, injection equipment, and personal protective equipment.
• Facilities should have at least one individual on staff trained in infection prevention. This individual should be involved in the development of written infection prevention policies and protocols. The development and ongoing refinement of infection prevention policies and procedures should be based on evidence-based guidelines, regulations, and standards. These policies and procedures should be tailored to the facility and re-assessed on a regular basis
• Educate and train healthcare personnel. Ongoing education and training of HCP to ensure that infection prevention policies and procedures are understood and followed. Education on the basic principles and practices for preventing the spread of infections should be provided to all HCP.
• Health association infection surveillance and reporting. At a minimum, facilities should adhere to local, state, and federal requirements regarding HAI surveillance, reportable diseases, and outbreak reporting. Facilities should check the requirements for their state/region to assure they are compliant with all regulations and should ensure required reporting is done promptly.
• Hand hygiene. As recommended by the World Health Organization (WHO) and the CDC, hand hygiene includes the use of alcohol-based hand rubs and soap and water, which is critical to reduce the risk of spreading infections in ambulatory care settings.
• Use of personal protective equipment. Personal Protective Equipment (PPE) refers to wearable equipment that is intended to protect HCP from exposure to or contact with infectious agents. Examples include gloves, gowns, face masks, respirators, goggles and face shields.
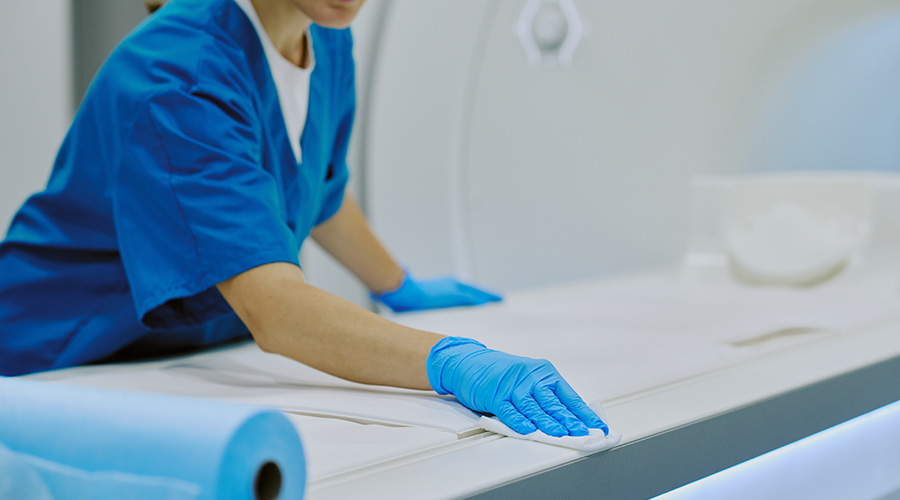
• Cleaning, disinfection, and sterilization of medical equipment. Facilities should ensure that reusable medical equipment (e.g., blood glucose meters and other point-of-care devices, surgical instruments, endoscopes) is cleaned and reprocessed appropriately prior to use on another patient. Reusable medical equipment must be cleaned and reprocessed (disinfection or sterilization) and maintained according to the manufacturer’s instructions.
• Respiratory Hygiene/Cough Etiquette Facilities should implement measures to contain respiratory secretions in patients and accompanying individuals who have signs and symptoms of a respiratory infection, beginning at point of entry to the facility and continuing throughout the duration of the visit (i.e., Post signs, offer masks, provide tissues and hand hygiene resources). Facilities should also educate HCP on the importance of infection prevention measures to contain respiratory secretions to prevent the spread of respiratory pathogens when examining and caring for patients with signs and symptoms of a respiratory infection.
• Cleaning and disinfection of environmental surfaces. Ambulatory care facilities should establish policies and procedures for routine cleaning and disinfection of environmental surfaces as part of their infection prevention plan.
• Safety injection practices. Injection safety includes practices intended to prevent transmission of infectious diseases between one patient and another, or between a patient and healthcare provider during preparation and administration of parenteral medications.
The recommendations above described by the CDC represent the absolute minimum infection prevention expectations for safe care in outpatient (ambulatory care) settings. This guidance is not all encompassing. It is recommended that facilities and HCP refer to the original source documents and maintain thorough processes.
Christine Queally Foisey is the President & CEO of MedSafe.

 Hand, Foot and Mouth Disease on the Rise
Hand, Foot and Mouth Disease on the Rise Preparing for the Hazards of Winter Weather
Preparing for the Hazards of Winter Weather BayCare Reveals Pagidipati Children's Hospital at St. Joseph's
BayCare Reveals Pagidipati Children's Hospital at St. Joseph's Why Identity Governance Is Becoming a Facilities Management Issue
Why Identity Governance Is Becoming a Facilities Management Issue Habitat Health Opens South Los Angeles PACE Center
Habitat Health Opens South Los Angeles PACE Center