As surgical procedures continue amid the COVID-19 pandemic, the need for safe and more efficient surgical tools has never been greater. During this time, hospitals might be looking for ways to simplify sterilization processes and limit infection exposure.
During the pandemic, it is critical that hospitals’ sterile processing departments (SPDs), the hub where surgical instruments are processed and reprocessed daily, ensure staff and patient safety. To do this, SPDs should be designed to maximize efficiency and adhere to industry standards set by groups such as the Association for the Advancement of Medical Instrumentation (AAMI), the Association of periOperative Nurses (AORN), the Society of Gastroenterology Nurses and Associates (SGNA) and The Joint Commission.
With this in mind, SPD designers should consider how they organize storage space, reduce touchpoints and minimize inspection time to make the sterilization process as safe and efficient as possible.
Organizing the storage space
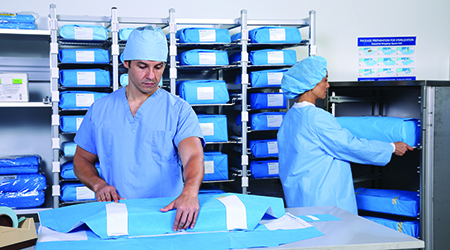
Effectively using storage space is a crucial step to improve efficiency. One way SPDs try to organize these spaces is by stacking sterilized trays on top of one another, which can lead to added handling and increased contamination risk.
Many SPDs operate under the impression that they have no choice but to stack sterilized packages due to space constraints. However, the constant stacking can lead to increases in tears, holes and cuts, which can contaminate the trays. A system in which wrapped, sterilized trays can be sorted into storage without being stacked, and left undisturbed until they are ready for use, is optimal to continue to ensure safety.
For example, the Halyard and Belintra Smart-Fold Sterisystem uses the available space in the SPD to increase storage space by incorporating a modular storage system that enables facilities to handle sterilized trays in a safe and efficient manner. By decreasing the unnecessary handling of packages, there are fewer opportunities to breach the sterility, which improves overall infection control and decreases the opportunities for contaminants to spread.
Reducing touchpoints
Reducing touchpoints, or the amount of times a sterilized package is handled, is an important consideration when streamlining the sterilization process and preventing the spread of viruses. SPD designers can consider different tools that can help reduce touchpoints to either meet or exceed the AAMI standard 79 guideline that designates three touchpoints to maintain sterilization.
However, when SPDs are organized with stacking sterilized trays, the touchpoints are increased for every tray that must be moved, and time is wasted stacking and restacking the trays. Eliminating stacking leads to fewer opportunities for contamination, ultimately helping to prevent contamination from potential pathogens.
Minimizing inspection time
A common bottleneck in SPDs is the inspection process of sterilized trays. Every tray must be thoroughly examined throughout the sterilization process, and the way an SPD is designed and which storage systems are chosen can result in a vast difference in the average time the inspection process takes.
Some SPDs use rigid sterilization containers – reusable metal containers with gaskets, seals and filters – while others use pliable sterilization wraps that are used to encase surgical trays. In a study published by the American Journal of Infection Control, sterilization wrap was shown to maintain 100 percent sterility post-sterilization vs. rigid sterilization containers. In fact, 87 percent of the rigid sterilization containers tested positive for bacterial contamination post-sterilization.
Sterilization wrap and rigid containers must undergo inspections to ensure sterilization is maintained in the SPD. With the rigid container system, every component of the container — top, bottom, filter, lock — must function as a unit in order to maintain sterility. As every single element of the system must be inspected, rigid containers can take more than 40 minutes for disassembly, automatic washing, reassembly and inspection.
Throughout this process, the integrity of the system – which might be compromised by dents, cracks, or mismatched container components – might not be immediately clear. In comparison, inspecting sterilization wrap for a potential breach involves identifying tears, cuts or holes and takes less than two minutes. This simpler process might help combat the spread of pathogens and provides the added benefit of potential cost savings that come from less inspection time.
The importance of streamlining
Organizing the storage space, reducing touchpoints, minimizing inspection time and choosing the right storage system should be among the many factors considered when designing an SPD. Implementing a system that can streamline an SPD, especially one in which stacking can be avoided, can not only improve the financial bottom line for a hospital. It also can reduce the chances for infection and the spread of viruses, ultimately improving staff and patient safety.
Joe Hannibal is senior marketing director with O&M Halyard.
 UF Health Hospitals Rely on Green Globes to Realize Their Full Potential
UF Health Hospitals Rely on Green Globes to Realize Their Full Potential How Healthcare Facilities Can Be Truly Disaster-Resilient
How Healthcare Facilities Can Be Truly Disaster-Resilient TriasMD Breaks Ground on DISC Surgery Center for San Fernando Valley
TriasMD Breaks Ground on DISC Surgery Center for San Fernando Valley Bigfork Valley Hospital Falls Victim to Data Breach
Bigfork Valley Hospital Falls Victim to Data Breach AI-Driven Facilities: Strategic Planning and Cost Management
AI-Driven Facilities: Strategic Planning and Cost Management