The Americans with Disabilities Act is most commonly associated with addressing roadblocks to accessibility into, through and outside of the nation’s institutional and commercial facilities – commercial offices, education facilities and government buildings, among them. From sidewalks and doors to elevators, hallways and restrooms, the law has laid out accessibility guidelines that facility managers have consulted in removing barriers to access for all.
Healthcare facilities also must comply with ADA’s requirements, and the U.S. Access Board is seeking input on improving one particular aspect of the guidelines.
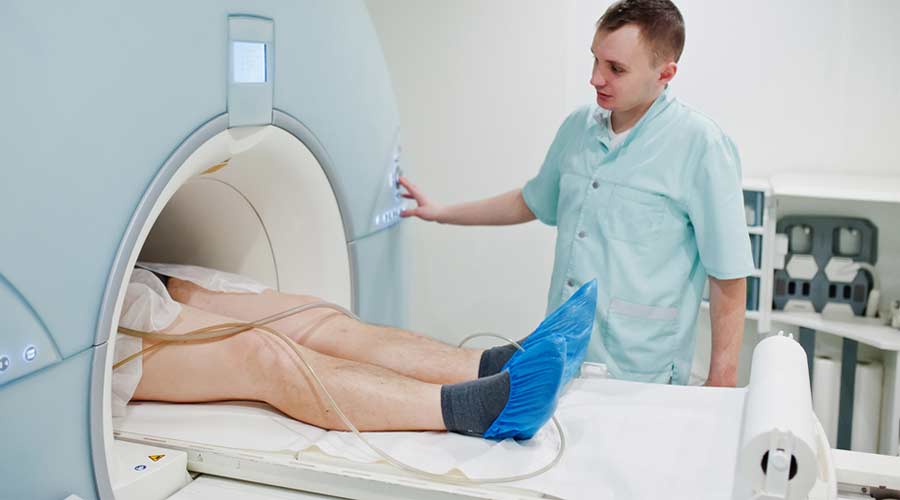
The board has an open comment period through May 27, 2022, regarding the appropriate low height of medical diagnostic equipment with transfer surfaces, including examination tables and chairs and diagnostic imaging medical equipment with tables, so that the equipment can be adjusted to accommodate the broadest range of users. The board continues to seek information on low transfer heights for adjustable medical diagnostic equipment products that are on the market and any changes or innovations in their design and engineering that might have occurred since the board issued its medical diagnostic equipment accessibility standards.
The board will use this information to assess specifications for transfer surfaces in its medical diagnostic equipment (MDE) accessibility standards. Published in January 2017, these standards address various features of MDE, including the range of adjustability of transfer surfaces. In the rulemaking, there was a lack of consensus on what the low height for transfer surfaces should be, and the board specified a temporary range of 17-19 inches with a sunset provision to allow time for further study. The sunset period was recently extended, and the board commissioned a statistical analysis to provide further insight into this issue. The report is available on the board’s website.
 UF Health Hospitals Rely on Green Globes to Realize Their Full Potential
UF Health Hospitals Rely on Green Globes to Realize Their Full Potential How Healthcare Facilities Can Be Truly Disaster-Resilient
How Healthcare Facilities Can Be Truly Disaster-Resilient TriasMD Breaks Ground on DISC Surgery Center for San Fernando Valley
TriasMD Breaks Ground on DISC Surgery Center for San Fernando Valley Bigfork Valley Hospital Falls Victim to Data Breach
Bigfork Valley Hospital Falls Victim to Data Breach AI-Driven Facilities: Strategic Planning and Cost Management
AI-Driven Facilities: Strategic Planning and Cost Management